Data - Information - Knowledge - Results
During the industrial revolution companies that can effectively manage their raw materials gain competitive advantage through their efficiencies. Take for example Carnegie Steel Company in the early 1900s. Carnegie Steel was the most successful company in its time because it was effective managing raw materials and the complexity of its businesses by being innovators in its industry. Now in the global information economy of the 21st century, effective information management is critical to gaining competitive advantage. In virtually all industries businesses collect, store, validate, analyze, and share data. Company that can best transform these data into information will have an edge in realizing their speed to insight. When data can be transformed into information, vast knowledge can be gain resulting in better and timely decision making.
In the Pharmaceutical industry there are many challenges to product development. This is an industry where intellectual property asset is the life blood of its survival.
Challenges in the pharmaceutical industry:
- Inefficient Drug Production – Inefficiencies at the clinical trial stages of drug production, including ineffective clinical development process and lengthy development cycles, significantly increase the development costs of new drugs.
- Long Trial Phase – The Human Clinical Trials phase of drug development is lengthy (5 – 7 years).
- Outdated Legacy Systems – Many drug manufacturers have multiple systems, some commercial, many legacy “home-grown”, typically based on Oracle / Unix systems, with little Microsoft penetration beyond the desktop. These systems are often overly complex and proprietary applications that do not allow a high level of data integration or scalability.
- Lack of System Integration and Consistent Application Platform– Integration is challenging because of multiple sources of data in locked silos with a lack of a shared information base, frequent duplication of information, and difficulty in making informed decisions. Data derived from internal and external sources must be integrated from disparate systems into the existing legacy systems.
- Profitability Pressure – Potential price controls, increased costs of developing new drugs, and the costs associated with regulatory compliance lead to a high pressure on profitability.
- Shortage of Human Capital Resources – Processes in place to manage human resources are too complex and inefficient. Low agility in HR results from a lack of resources and communication across the clinical development landscape.
- Competition – There is an increasing number of potential drug candidates without an increasing supply of qualified staff and resources.
Lack of data integration forces pharmaceutical companies to introduce many manual measures into the overall clinical trial processes to ensure quality of data, compliance to regulatory mandates, and provide quality patient care. In late phase clinical trials, shortening cycle time is critical to being competitive in this highly regulated industry, especially in “block-buster” drugs. Huge opportunities exist to leverage information technology in ways that would shorten the lifespan of clinical trials without sacrificing quality. So what can we do to address these business imperatives and technical challenges with data integration?
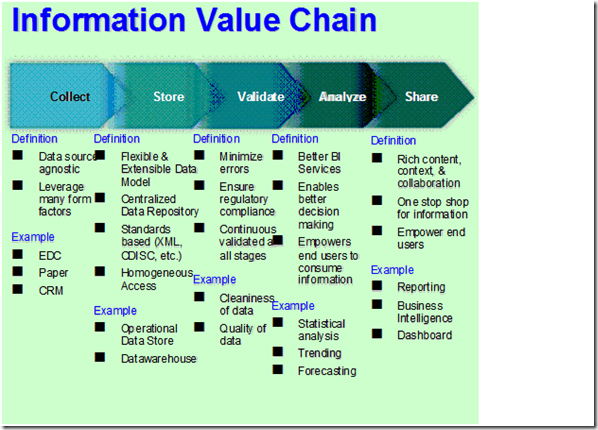
If we break down the fundamental components of data integration challenges, we would see the following information value chain; Collection of Data, Storage of Data, Validation of Data, Analysis of Data, and
Sharing of Data. See Figure 1.
Figure 1: Information Value Chain
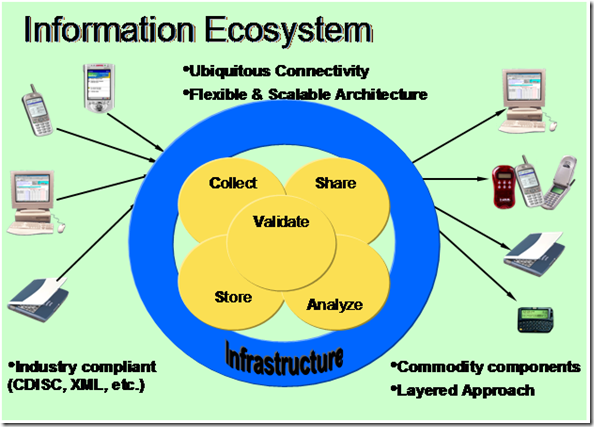
Off course the information value chain is only one major component of the Information Ecosystem. In order for pharmaceutical companies to capitalize and realize efficiencies; information standards and architecture standards must be define to ensure common implementations.
In the Information Ecosystem, the following characteristics are important.
- Ubiquitous connectivity in and out of the system – Standard way of communicating with other systems both internal and external to itself (i.e. Web Services, etc.)
- Common Architectural Framework that is flexible and scalable - (i.e. SOA, Microsoft Digital Pharma Framework)
- Adherence and support for industry standards (i.e. CDISC, XML, HL7, etc.)
- Build upon known components and services – (i.e. Build from assembly instead of from scratch)
Information collected, stored, validated, analyzed, and shared are created by end users and consumed by end users via the same access mechanisms for example, Desktop computers, Tablet PCs, and PDA.
See Figure 2: Information Ecosystem
One could make the case that these fundamental activities are common to all industries in addition to the Healthcare and Life Sciences industry. For example companies need to manage their information system to as a naturally consequence of doing business. In the Automotive Industry, Ford Motor Company would transform information into the cars and trucks that we drive. In the Life Sciences industry; pharmaceutical companies such as Merck would transformed information into the drugs we consumed.
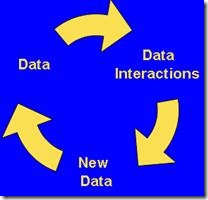
In all cases data integration is a key component in effectively management the Information Ecosystem. In the Pharmaceutical industry as in most other industry, data are constantly being collected. The actions and decisions performed on these data are then collected to form new data. This constant data interactions is a natural occurrence as a by product of normal interactions between the consumers of the data and the data itself. See Figure 3 – Data Interaction.
Figure 3: Data Interaction
Comments
Anonymous
December 29, 2007
PingBack from http://cars.oneadayvitamin.info/?p=775Anonymous
June 25, 2011
infomation ecosstem is a good picter